Phosgene and phosgenationProcess
Reaction type
Exothermic reaction
Key monitoring unit
Phosgenation reactor, phosgene storage and transportation unit
Process introduction
The phosgene and phosgenation process includes the preparation process of phosgene and the process route for preparing phosgenation products using phosgene as raw material. The phosgenation process is mainly divided into two types: gas phase and liquid phase.
Hazardous characteristics of the process
(1) Phosgene is a highly toxic gas, which can easily cause large-scale pollution and poisoning accidents after leakage during storage, transportation and use;
(2) The reaction medium has the risk of explosion;
(3) The by-product hydrogen chloride is corrosive and can easily cause equipment and pipeline leaks and poisoning accidents to personnel.
Typical process
The reaction of carbon monoxide and chlorine produces phosgene; phosgene synthesizes diphosgene and triphosgene;
Synthesis of polycarbonate using phosgene as monomer; Preparation of toluene diisocyanate (TDI);
Preparation of 4,4′-diphenylmethane diisocyanate (MDI), etc.
Focus on monitoring process parameters
The water content of carbon monoxide and chlorine; the temperature and pressure of the reactor; the batching ratio of the reaction materials; the feed rate of phosgene; the temperature, pressure, and flow rate of the cooling medium in the cooling system, etc.
Basic requirements for security control
Accident emergency shut-off valve; emergency cooling system; reactor temperature and pressure alarm interlock; local exhaust facilities; toxic gas recovery and treatment system; automatic pressure relief device; automatic ammonia or alkali spray device; phosgene, Chlorine, carbon monoxide monitoring and over-limit alarm; dual power supply
The appropriate control method
Once abnormal phenomena occur in the phosgene and phosgenization production system or a leakage accident of phosgene and its highly toxic products occurs, the emergency stop should be initiated through the automatic interlocking device and all materials entering and exiting the production device should be automatically cut off, and the reaction device should be Rapidly cool down, and at the same time, introduce the highly toxic materials in the equipment where the accident occurred into the accident tank, start spraying ammonia water and dilute alkali solution, start the ventilation and detoxification system, and discharge toxic gases from the accident site to the treatment system.
2 Electrolysis process (chlor-alkali)
Reaction type
Endothermic reaction
Key monitoring unit
Electrolyzer, chlorine gas storage and transportation unit
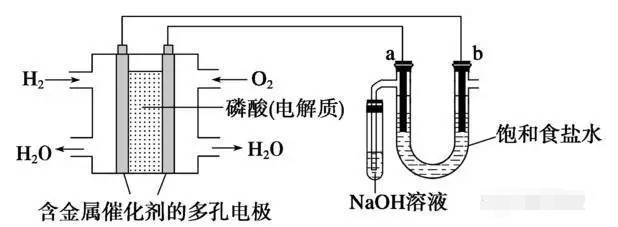
Process introduction
When an electric current passes through an electrolyte solution or molten electrolyte, the chemical changes caused on the two poles are called electrolysis reactions. The process involving electrolysis reaction is electrolysis process. The preparation of many basic chemical industry products (hydrogen, oxygen, chlorine, caustic soda, hydrogen peroxide, etc.) is achieved through electrolysis.
Hazardous characteristics of the process
(1) The hydrogen gas produced during the electrolysis of salt water is an extremely flammable gas, and chlorine gas is a highly oxidizing and highly toxic gas. The mixture of the two gases is prone to explosion. When the hydrogen content in the chlorine gas reaches 5% Above, it may explode at any time when exposed to light or heat;
(2) If the ammonium salt present in the brine exceeds the standard, under suitable conditions (pH<4.5), the action of ammonium salt and chlorine can produce ammonium chloride, and concentrated ammonium chloride solution and chlorine can also produce yellow oily Nitrogen trichloride. Nitrogen trichloride is an explosive substance that will decompose violently and explode when it comes into contact with many organic substances or is heated to above 90°C, or is hit or rubbed;
(3) The electrolytic solution is highly corrosive;
(4) Leakage of liquid chlorine may occur during the production, storage, packaging, transportation, and transportation of liquid chlorine.
Typical process
Electrolysis of sodium chloride (salt) aqueous solution to produce chlorine, sodium hydroxide, and hydrogen;
Electrolysis of potassium chloride aqueous solution produces chlorine, potassium hydroxide, and hydrogen.
Focus on monitoring process parameters
The liquid level in the electrolytic cell; the current and voltage in the electrolytic cell; the flow of materials in and out of the electrolytic cell; the concentration of flammable and toxic gases; the temperature and pressure of the electrolytic cell; the ammonium content in the raw materials; the content of chlorine impurities (water, hydrogen, oxygen, Nitrogen trichloride, etc.) etc.
Basic requirements for security control
Alarms and interlocks for electrolyzer temperature, pressure, liquid level and flow rate; alarms and interlocks for electrolysis power supply rectifier and electrolyzer power supply; emergency interlock cut-off devices; chlorine gas absorption and neutralization system under accident conditions; flammable and toxic gasDetection and alarm devices, etc.
The appropriate control method
The pressure inside the electrolytic cell, cell voltage, etc. form an interlocking relationship, and the system establishes an interlocking parking system.
Safety facilities include safety valves, high-pressure valves, emergency discharge valves, liquid level gauges, one-way valves and emergency shut-off devices, etc.