Urethane elastomerProduction process
1. Overview of polyurethane elastomer
The so-called elastomer refers to a polymer material with a glass transition temperature lower than room temperature, an elongation at break of >50%, and good recovery properties after external force is removed. Polymer materials with a glass transition temperature higher than room temperature are called plastic. Among elastomers, those with larger elongation at break (>200%), smaller 100% elongation stress (such as <30Mpa), and better elasticity can be called rubber. Therefore, elastomers are a broader class of polymer materials than rubber.
Polyurethane rubber, also known as polyurethane rubber, is a relatively special category of elastomers. It has a wide variety of raw materials, various formulas, and a wide adjustable range. The hardness range of polyurethane elastomers is very wide, ranging from low modulus rubber below Sauer A10 to high impact rubber elastic materials such as Sauer D85. Therefore, the performance range of polyurethane elastomer is very wide, and it is a type of polymer material ranging from rubber to plastic.
2. Main raw materials of polyurethane elastomer
The raw materials used in polyurethane elastomers mainly fall into three categories, namely oligomer polyols, polyisocyanates and chain extenders (cross-linking agents). In addition, sometimes in order to increase the reaction speed, improve processing performance and product performance, certain compounding agents need to be added. Only the raw materials used in the production of polyurethane saddles are described in detail below.
Reaction process: polyol reacts with diisocyanate to form a low molecular weight prepolymer; through chain extension reaction, a high molecular weight polymer is formed; then an appropriate cross-linking agent is added to form a polyurethane elastomer. The process flow is as follows:
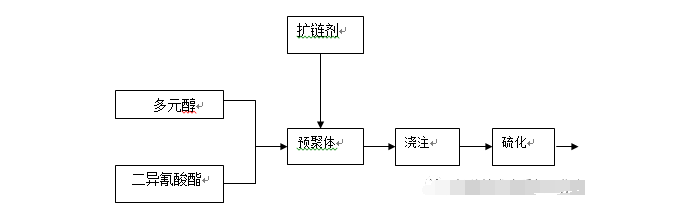
2.1 Oligomer polyol
The average functionality of oligomer polyols used in polyurethane is low, usually 2 or 2~3. The relative molecular mass is 400~6000, but the commonly used ones are 1000~2000. The main categories are polyester polyols, poly Ether polyols, polyε-caprolactone diols, polybutadiene polyols, polycarbonate polyols and polymer polyols, etc. They play a very important role in the synthesis of polyurethane resins. Generally, the physical and chemical properties of polyurethane can be adjusted by changing the type, molecular weight, functionality and molecular structure of polyol compounds.
2.1.1 Polyester polyol
Polyester polyol, referred to as polyester, is one of the most important raw materials for polyurethane elastomer. It is formed by the condensation polymerization of dicarboxylic acid and polyol. The most commonly used dicarboxylic acid is adipic acid. The most commonly used polyols are ethylene glycol, propylene glycol, butylene glycol and diethylene glycol. In addition, some special polyesters also use polyols such as pentanediol, ethylene glycol, trimethylolpropane, and glycerol. Since there are many types of polyols available, the molecular structures of polyester are diverse and there are many varieties and brands. In order to obtain hydroxyl-terminated polyester, excess polyol must be reacted with dicarboxylic acid. Polyester is generally produced using the intermittent method. The reaction process is divided into two stages: esterification reaction and transesterification reaction. The production equipment is similar, the general process flow, and the main equipment include condensation kettle, fractionation condenser, condenser, metering tank, vacuum system, heating and cooling system and control system. The air tightness requirements of the entire system are very strict. The stirring shaft of the condensation kettle can adopt end mechanical seal. The feeding sequence is to add polyol and compounding agent first, then add adipic acid, and then fill with nitrogen. The esterification reaction starts from heating to 220~250°C and is basically completed in about 1 hour. This stage is mainly a normal pressure dehydration process to generate low molecular weight polyester and condensation water. When the temperature rises to about 135°C, the esterification reaction becomes most intense and a large amount of condensation water is generated. Due to the evaporation of condensed water, a large number of bubbles will rise, and the bubbles are particularly intense when 1,4-butanediol and 1,6-ethylene glycol are used as raw materials. At this time, the heating power should be adjusted in time to control the water outlet speed of the condenser to prevent a large amount of water vapor from taking a large amount of molecular polyol out of the fractionation condenser. After the intense reaction, maintain an appropriate water output rate and gradually increase the reaction temperature to 220~250°C. When the acid value drops to about 30mgKOH/g or the water output is approximately equal to the theoretical water amount, because there are very few hydroxy acids in the mixture, esterification It is difficult to continue, the water output basically stops, and the esterification reaction stage is basically completed.
The oligomer polyol selected for this polyurethane saddle is polyester polyol, with the brand name ODX-218 and a molecular weight of 2000.
2.2 Polyisocyanate
There are many varieties of polyisocyanates, but only two are the most produced, namely diphenylmethane diisocyanate (MDI) and its polymer polyphenyl polymethylene polyisocyanate (PAPI) and toluene diisocyanate (TDI) ). As polyisocyanate, we use TDI-100 from Bayer of Germany.
TDI uses toluene as the basic raw material. It is nitrated with a mixture of nitric acid and sulfuric acid to generate dinitrotoluene, which is then dissolved in methanol and hydrogenated and reduced to dinitrotoluene under a Raney catalyst and a hydrogen pressure of 15~20Mpa. Amine (TDA), produced by phosgenation. </pcan only be used.
5. Polyurethane elastomer production process
Polyurethane elastomers are generally manufactured using two process routes: prepolymerization and one-step method. Casting elastomers mostly use a two-step method (prepolymer method), and a few use a one-step method (such as low modulus products).
Casting polyurethane elastomer (CPU for short) – is the most widely used and most produced type of polyurethane elastomer; it can be cast and poured into products that can be poured into various complex molds.
5.1 One-step synthesis of prepolymers
The one-step synthesis of polyurethane casting glue (CPUR) is to put polymer diol, diisocyanate and chain extender together, mix them thoroughly and then pour them into a mold to be heated and solidified. After the size is stable, post-vulcanization is performed. The post-vulcanization temperature condition is 3-24h at 100℃, as shown in the figure below:
One-step synthesis of CPUR generally has poor physical properties. Only when the hydroxyl value of the polymer polyol is >2, or the -NCO number of the polyisocyanate is >2, the one-step synthesis is most suitable, such as soft foam plastics and hard plastics. Foam plastics, etc., using a one-step method. For raw materials with polymer polyols and polyisocyanates with hydroxyl numbers equal to 2, it is best to use prepolymers to synthesize CPUR.
5.2 Synthesis of two-step prepolymer
When making larger polyurethane products, simply using polyisocyanate and polymer polyol to react in one step will release a lot of heat, causing the inside of the product to be heated and aged. At the same time, it will decompose and release low molecular substances, causing foam to form inside the product. Become waste. Therefore, extra large cast polyurethane products cannot be produced using the one-step method. Polyurethane casting rubber products are synthesized by prepolymerization method, and the operation is smooth during the production process without overheating. Therefore, this product is synthesized using a two-step method (prepolymerization method).
Put the prepolymers made from polymer diol and diisocyanate together and mix them thoroughly. After vacuum degassing, they are injected into the mold, injected into the mold, solidified, and then vulcanized to obtain the product. See the figure below for details:
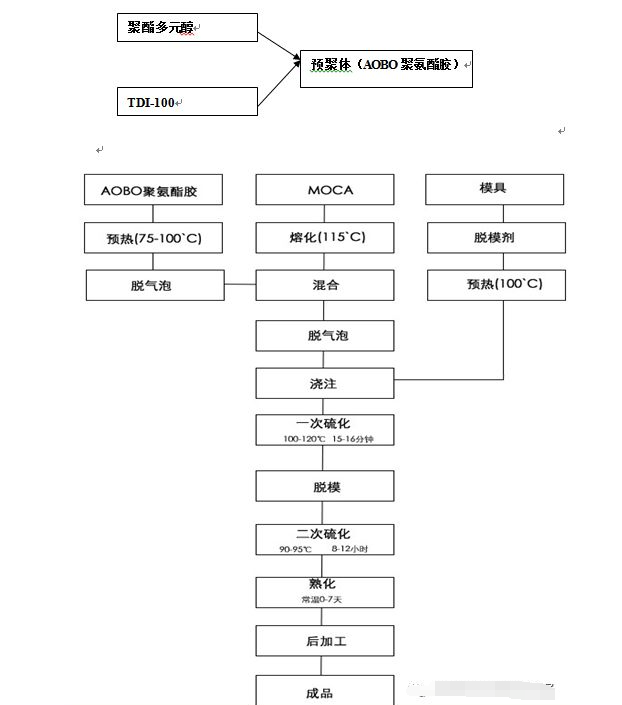
Note: Users can increase or decrease the procedures appropriately according to their own operating conditions and experience.
To ensure improved production efficiency or improved product accuracy.
First, the polyester is dehydrated under reduced pressure at 130°C. The dehydrated polyester raw material (at 60°C) is added to the reaction vessel containing the blended amount of TDI-100, and the prepolymer is synthesized with sufficient stirring. The synthesis reaction is exothermic. Care should be taken to control the reaction temperature within the range of 75°C-82°C, and the reaction can last for 2 hours. The synthesized prepolymer was then placed in a 75°C vacuum drying oven and vacuumed and degassed for 2 hours before use.
Then the prepolymer is heated to 100°C and evacuated (vacuum degree -0.095mpa) to remove bubbles. The cross-linking agent MOCA is weighed and heated to 115°C with an electric furnace to melt it. The mold is coated with a suitable release agent to preheat. (100°C), mix the degassed prepolymer and melted MOCA at a mixing temperature of 100°C, and stir evenly. Vacuum the evenly stirred mixture again to remove bubbles, and quickly remove the bubbles after stirring evenly. Pour into the preheated mold and when the mixture does not flow or stick to your hands (gel-like), close the mold and place it in a vulcanizer for molding and vulcanization (vulcanization conditions: vulcanization temperature 120-130°C, vulcanization time, For large and thick elastomers, the vulcanization time is more than 60 minutes; for small and thin elastomers, the vulcanization time is 20 minutes). After vulcanization treatment, the molded and vulcanized products are placed at 90-95°C (under special circumstances, they can be 100℃), continue vulcanization in the oven for 10 hours, and then leave it at room temperature for 7-10 days to complete the curing, and finally the finished product is made.
Based on the above processing process, it can be deduced that the daily production capacity of a pair of polyurethane elastomer molds can reach up to 8 pieces/day.
6. Key operating points during polyurethane elastomer processing
6.1 Temperature control
When synthesizing the prepolymer, the temperature should be controlled between 75-82°C. If it is higher, the performance of the synthesized prepolymer will be reduced, and if it is lower, the polymerization time will be lengthened. The prepolymer temperature when mixed with MOCA is controlled between 90-110°C. If it is higher, it will reduce the hardness and strength of the product, and if it is lower, it will increase the viscosity of the polymer, which is not conducive to pouring operations. The vulcanization time is controlled at 130°C. If it is too high, MOCA will decompose, which is not conducive to the cross-linking reaction and will increase other side reactions; if it is too low, it will prolong the molding time. The melting temperature of MOCA should be controlled just after it melts into a liquid. Do not continue to heat it, otherwise the color of the liquid MOCA will darken and decompose, affecting the performance of the product.
6.2 Time Control
The synthesis reaction lasts for 2 hours, and the intermittent vacuuming is sufficient for 2 hours. Then seal it and store it at room temperature. Prepolymer synthesis should not exceed 4 hours at 75°C, let alone 2 hours at high temperature of 100°C, otherwise the performance of the product will be reduced. Pouring should be completed within 1-2 minutes, because the stability period of the prepolymer and MOCA is very short after mixing, generally only 4-5 minutes, otherwise the mixture will solidify and pouring will not be possible. The molding vulcanization time depends on the shape and size of the product, generally between 15-30 minutes, to ensure that the product is molded. For large products, the time will be longer, 2-3 hours.
6.3 Degassing of prepolymer
The quality of degassing is the key to the success of cast polyurethane elastomers. Generally, two steps need to be controlled. After the prepolymer is synthesized and left to stand, it is vacuum degassed at 75°C to remove most of the gas in the prepolymer. Before mixing the prepolymer with MOCA, it needs to be heated to 100-110°C. Degas; at the same time, vacuum (-0.095mpa) to degas for 10 minutes, then take it out and stir it to flip the bottom bubbles to the top, and then vacuum and degas for 10 minutes. After the gas in the prepolymer is completely discharged, it can be mixed with the vulcanizing agent to cast the product.
Certify product molding. For large products, the time will be longer, 2-3 hours.
6.3 Degassing of prepolymer
The quality of degassing is the key to the success of cast polyurethane elastomers. Generally, two steps need to be controlled. After the prepolymer is synthesized and left to stand, it is vacuum degassed at 75°C to remove most of the gas in the prepolymer. Before mixing the prepolymer with MOCA, it needs to be heated to 100-110°C. Degas; at the same time, vacuum (-0.095mpa) to degas for 10 minutes, then take it out and stir it to flip the bottom bubbles to the top, and then vacuum and degas for 10 minutes. After the gas in the prepolymer is completely discharged, it can be mixed with the vulcanizing agent to cast the product.