Why can LiCl accelerate the formation of organozinc reagent
In order to explore the mechanism of a certain chemical reaction, people have developed more and more detection methods for analysis and verification. With the increasing number and complexity of reaction types, existing analytical methods sometimes cannot meet people’s needs for mechanism research. For example, Professor Paul Knochel of the University of Munich in Germany has made important contributions in the research of organometallic reagents (such as Grignard reagents and organozinc reagents). In 2006, he discovered that LiCl could accelerate the insertion of halogenated hydrocarbons into metal Zn to form organozinc reagents. Previously, only Grignard reagents could be prepared by directly inserting halogenated hydrocarbons into metal elements. This discovery subsequently led to the efficient synthesis of a series of other organometallic reagents, such as organindium, organomanganese, organoaluminum reagents, etc.
However, people do not have a clear understanding of the role of LiCl in accelerating the formation of organozinc reagents and the changes in the structure of organozinc reagents. Previous studies have speculated that the possible effects of LiCl are as follows: (1) LiCl can promote the dissolution of the formed organozinc reagent, effectively exposing the zinc metal surface to continue participating in the reaction; (2) LiCl can complex with the aromatic ring of halogenated aromatic hydrocarbons Its electrophilic activation promotes the electron transfer process; (3) LiCl solution has high ionic strength, which can promote charge separation and accelerate metal insertion. However, these assumptions were not confirmed by corresponding experiments.
Recently, Professor Suzanne A. Blum of the University of California, Irvine, USA combined single metal particle fluorescence microscopy and nuclear magnetic resonance 1H spectroscopy to explain this problem. The sensitivity of single metal particle fluorescence microscopy is as high as the single molecule level, which can provide important information about intermediates formed in elementary reactions and overcome the limitations of insufficient sensitivity in monitoring reactions by other analytical methods in the past. Nuclear magnetic resonance spectroscopy analysis provides information on the overall reaction rate and product structure. The combination of the two can determine the influence of different lithium salts on each basic step of synthesizing organometallic reagents and the structure of organozinc reagents in solution, thus laying the foundation for further expansion of similar salt promotion effects and other types of organometallic reagents. important foundation. Relevant work was published in the well-known chemical journal J. Am. Chem. Soc.
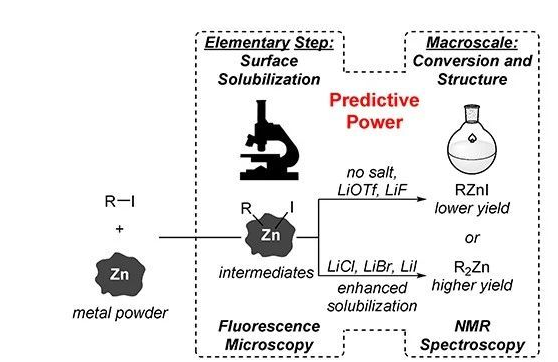
▲Single metal particle fluorescence microscope combined with NMR 1H spectroscopy technology (picture source: reference [1])
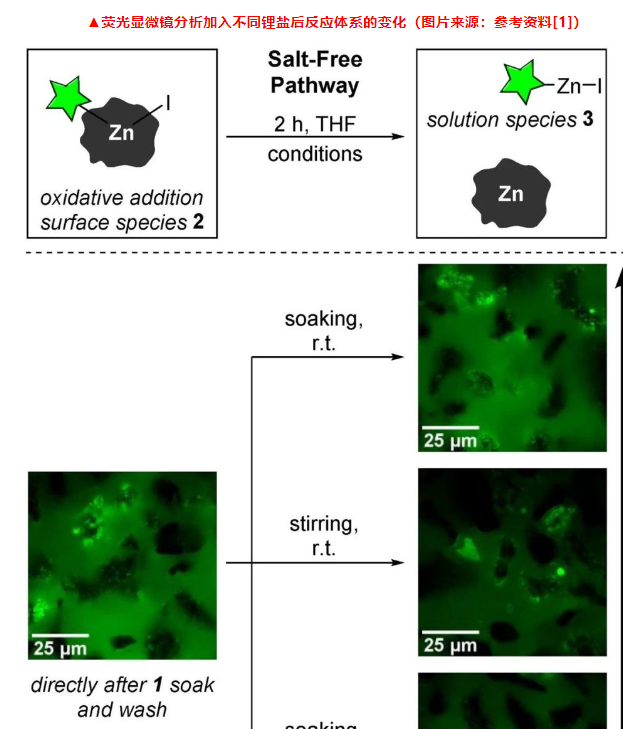
The author first designed a single-particle fluorescence microscope characterization experiment, using iodobutane (1) that modified the fluorescent probe structure as an oxidative addition imaging agent. The fluorescent luminescent structure is boron fluoride complexed dipyrromethene (BODIPY), which can be used to mark and track the reaction site where iodobutane is inserted into the Zn element to form the oxidative addition surface intermediate (2). When 1 does not participate in the reaction, it diffuses rapidly in the solution state, so no fluorescence imaging is observed. However, when it oxidizes and adds to Zn to form 2 on the metal surface, the fluorescent luminescent group remains stationary without mechanical disturbance. At this time Bright green “hot spots” appear on the surface of non-luminous zinc particles.
▲Single metal particle fluorescence microscopy analysis method flow chart (picture source: reference [1])
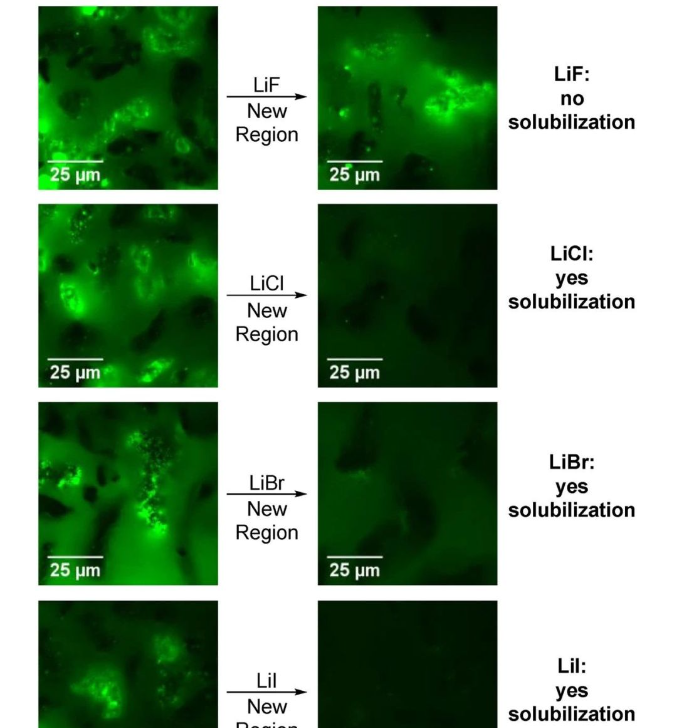
Without adding any lithium salt, the bright green 2 exists stably, but after adding LiCl, it quickly dissolves and the fluorescence disappears. The author also investigated the effects of other lithium halide (LiX, They also stirred and heated the system separately without adding any lithium salt. The experimental results showed that the former can partially dissolve 2, and the latter can significantly dissolve 2, but the dissolution rate is significantly slower than LiX (X = Cl, Br, I) exists. This important information cannot be obtained through traditional detection methods, which reflects the great advantages of fluorescence microscopy technology in terms of sensitivity and spatial positioning.
Picture ▲ Fluorescence microscope analysis of changes in the reaction system after adding different lithium salts (Picture source: Reference [1])
▲Fluorescence microscope analysis of the impact of different operations on the reaction system without adding lithium salt (Picture source: Reference [1])
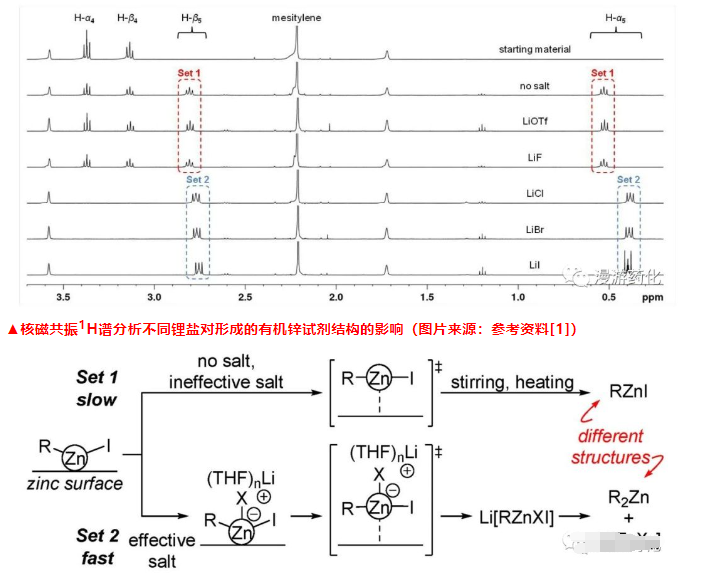
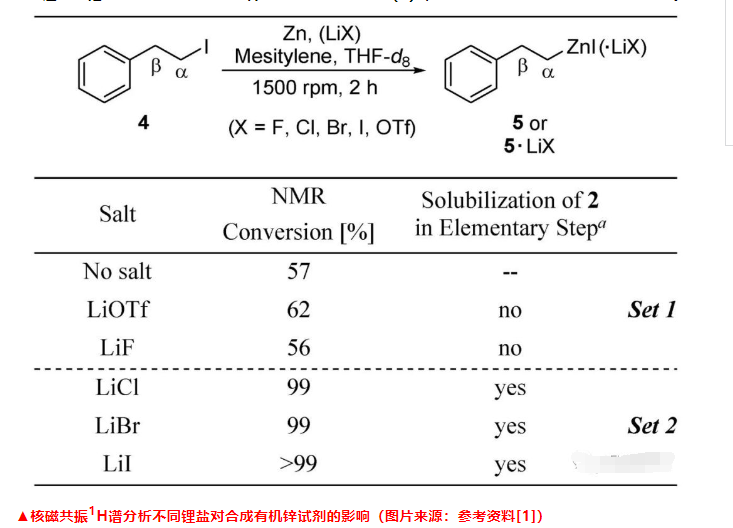
In order to verify whether the results observed by fluorescence microscopy can effectively predict which salt can promote the synthesis of organozinc reagents, the author further designed a nuclear magnetic resonance 1H spectrum experiment, using (2-iodoethyl)benzene (4) as Template substrate and study its conversion into the corresponding organozinc reagent (5). They found that the conversion rate was lower when no lithium salt was added and when LiX (X = F, OTf) was added (denoted as group 1), while an almost quantitative conversion occurred when LiX (X = Cl, Br, I) was added reaction (recorded as group 2). It can be inferred that the lithium salt of Group 2 can dissolve the oxidative addition surface intermediate (2) formed on the surface of zinc metal, which is an important reason for accelerating the formation of organozinc reagents.
At the same time, they also found through NMR 1H spectrum analysis that the chemical shifts and peak shapes of the 1H characteristic peaks of the α-position methylene of the organozinc reagent obtained by the two groups of experiments were significantly different, and they had different structures in the solution. The organozinc reagents obtained in the first set of experiments were all RZnI, while those in the second set of experiments were mainly R2Zn. Based on the above experimental results, the author proposed a theory and prediction model for lithium salt to promote the formation of organozinc reagents.
So far, Professor Suzanne A. Blum has experimentally confirmed the reason why LiCl accelerates the formation of organozinc reagents, and demonstrated the important role of combining different analytical methods in exploring the reaction mechanism. Compared with traditional analysis methods, fluorescence microscopy technology shows irreplaceable advantages in detection sensitivity. Looking back at the unresolved issues in previous mechanism studies, people may consider using this method to design reasonable reaction models for further exploration.